
Many years ago I spent some weeks in the Adirondack Mountains as spring-blooming mountain laurel (Kalmia latifolia, Zone 5) spread delicate pink blossoms across broad slopes as far as the eye could see. It was a gorgeous sight, and I cut armloads of flowering branches for every room. I was so enthused with the beauty of these plants that I planted several in my garden at home. Sad to say, they languished and died, victims of my alkaline soil. Had I taken time to research the plant’s soil requirements, I would have learned that it’s an ericaceous plant like heather and rhododendron, and these all require acid soil.
There are dozens of named Kalmia cultivars, and I want to try again. But this time, I’m going to acidify the soil so that these beautiful plants will stay healthy and bloom lavishly each May. This is definitely not low-maintenance gardening.
The acid or alkaline register of soils (referred to as soil pH) is dictated by underlying bedrock. Limestone sections of bedrock will be covered with alkaline (high pH) soil; granite bedrock will be covered by acidic (low pH) soil. Soil pH can be changed for brief periods of time, perhaps four to six months, before the true pH register returns.
The addition of lime will raise the pH of an acidic soil; and the addition of sulphur will lower the pH of an alkaline soil. A soil-testing service will tell you how to take soil samples, and provide detailed analysis of pH and mineral content. But it’s not really necessary to know your soil’s exact pH. A simple home pH test purchased from a garden centre will provide a basic evaluation of soil acidity or alkalinity. My garden soil registers as slightly alkaline, and that’s bad news for growing mountain laurel.
I’ll dig in sulphur to acidify the soil when I plant the shrubs, and at least once every growing season. The amount of sulphur required depends on soil quality. Clay soil and soil rich in organic materials have a natural buffering ability and will require more sulphur; sandy soil requires less sulphur (follow package directions for application rates).
The ability of sulphur to lower soil pH is accomplished by microbial activity. Soil microbes are less active in cold soils, so the best time to dig in sulphur is in late spring through summer. Botanical grade, quick-release powdered sulphur is available in small amounts at garden centres. Farm-supply stores stock both powdered and slow-release pelletized sulphur in bulk bags.
Mountain laurel is an ideal companion plant for rhododendrons, requiring the same cultural conditions — part shade to sun, and consistently moist, acidic soil in the range of pH 5 to 6. It has similar foliage to rhododendron, but unlike rhododendrons, which carry their flower buds through winter, mountain laurel shrubs don’t produce their flowers buds until spring. The flowers are formed in dense corymbs, and are in the pink to raspberry, deep red and cinnamon-maroon shades (often with fancy white markings).
I wouldn’t want to alter the pH for more than a plant or two, but the showy flowers of mountain laurel are worth the additional maintenance effort. I’ve ordered the pretty new cultivar K. latifolia ‘Kaleidoscope’ (hortico.com) with dark red buds opening to cinnamon-red flowers with white edges. In my Zone 6 garden it could possibly grow to four feet (1.2 m) high and wide, although in warmer climates the shrubs can be much larger. There are several dwarf cultivars available, and I wouldn’t mind finding ‘Minuet’ (K. latifolia ‘Minuet’, Zone 6), 40 by 40 inches (1 by 1 m).
If you see any of these beautiful mountain laurel shrubs for sale, I’d be glad to know about it!


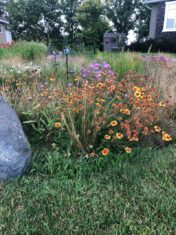






Hello. I am having a bit of a struggle with the Laurels that are growing in my garden. They are placed in the appropriate sun/shade regimen, the soil is kept moist and I fertilize according to the specifications. But late last summer and over this winter, a white, powdery substance started to form on a large number of the stems. In spite of efforts to try to revitalize and fertilize, the powdery substance consumed one of the Laurels which was quite sad. I had to dig it up about a week ago. Now, the two Laurel’s that were on either side of the late shrub have the same substance growing on the stems and I’m afraid that it will consume these as well.
Do you know what could be causing this to form and how I can get rid of this disease? I’ve read about powdery mildew and have been using a Bayer product to try to get rid of it…but nothing has worked as of yet. My neighbor and I have been trying to work on these together to figure out a solution and after speaking to a garden center, they said that the soil may not be acidic enough for the Laurels.
I’m just not sure what to do and I definitely do not want to lose the 5 other Laurels that are surrounding my porch area. Do you have any suggestions for me?
Thank you!!
Nick
Hi Nick, can you tell us where you live? That might help to identify a solution.
Hi Judith. How much of an effect, relative to the other factors you mention, do coniferous trees have on soil acidity? When out West I so often see rhodos in pine forests for example. I have spruce and cedar in my front garden so am hoping that it would be a natural home for these gorgeous mountain laurels. FYI that I’ve never tested the soil (new home to me with established garden). Your thoughts, or those of any other readers? Thank-you.
Margaret,
I wish it was that easy! Pine needles have a pH of about 3.2 to 3.6 when they fall from the tree. They’re slow to decompose, and if left to compost on the soil surface contribute no acidity to the soil. If conifer needles are dug into the soil, their slow decomposition releases acidity only in minute amounts over a long period of time. Unfortunately, rhododendron and kalmia won’t benefit from the acidity in these needles. However, conifer needles make good surface mulch around plants, and are excellent for aeration (boosting the oxygen content of soil) when dug in.
If you see pine trees and ericaceous plants growing together, their natural soil pH is sufficiently acidic to provide the pH they require.
— Judith